I'm thinkin' tank internal electroplating is viable. A long nickel anode (possibly a wire?) inside a plastic cage of some sort to prevent shorting against the tank. I think it's doable.
-
Enjoy XS650.com? Consider making a donation to help support the site.
XS650.com receives a small share of sales from some links on this page, but direct donations have a much greater impact on keeping this site going.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Heavy Metal. Rust Removal and plating
- Thread starter Jim
- Start date
Not worried about looks inside the tank, have seen people on UTube using nickel TIG rods. Rubber bung in the filler neck, TIG rod bent down each side of the tunnel, fill tank with solution...might just work eh?
Jim, electroplating does work in tanks. I major issue as pointed out by you above is the anode design to give good/uniform current density throughout the tank, especially on both sides in the case of a Special where the petrol cap is offset. Below is a link to a guidebook from the Nickel Institute:
https://www.nickelinstitute.org/media/2323/nph_141015.pdf
This is written for the layman and is easy going. Its speaks of such things as low chloride levels, pH control, anode shape, anode purity,current density, plating for corrosion resistance, troubleshooting etc etc. Yes, Quite a nice little 60 -70 page help guide.
https://www.nickelinstitute.org/media/2323/nph_141015.pdf
This is written for the layman and is easy going. Its speaks of such things as low chloride levels, pH control, anode shape, anode purity,current density, plating for corrosion resistance, troubleshooting etc etc. Yes, Quite a nice little 60 -70 page help guide.
Little? Yup. Light reading, for sure.Yes, Quite a nice little 60 -70 page help guide.
 The intellectual level on this site never ceases to amaze me. I stand in the shadow of giants. (Actually, I stand around the corner from the shadow of giants...
The intellectual level on this site never ceases to amaze me. I stand in the shadow of giants. (Actually, I stand around the corner from the shadow of giants...  )
)So.... for the money...
These are handlebar clamps from a DT250 project I haven't started... at least not in earnest yet. I bead blasted the rust off 'em about 4 mo. ago.

A couple spots reappeared, but no matter. Hit 'em with an 80G rolok sanding disk to sand the casting marks off. Then switched to a brown, then red then blue scotchbrite rolok disks. Then on to the buffer with brown rouge. If I had some white, I'd have used that too 'cause the brown left some marks... anyway, onward and upward.

Scrubbed them with dish soap, then a 5 min bath in lacquer thinner.

... then into the plating tank. Every couple minutes I stirred the solution and shook the bubbles off.
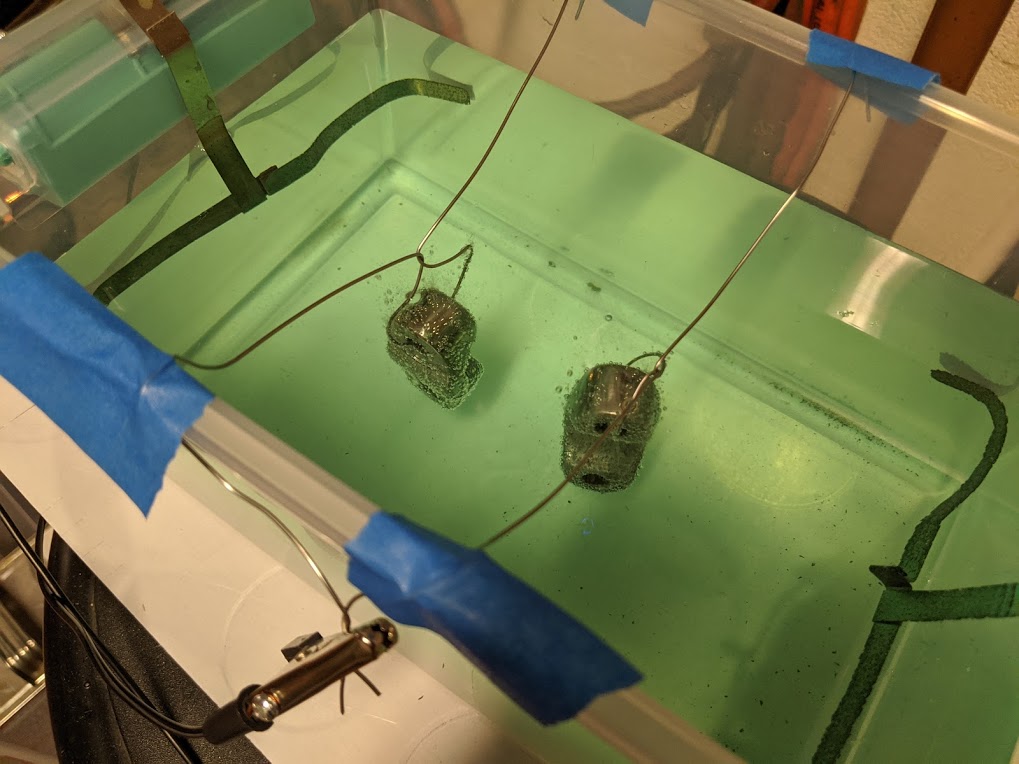
I want a nice thick plating, so I'm shooting for an hour. I'm watching closely for any signs of burning or blackening. As long as it stays bright... I'll keep going.
60 min later and here we are.


I'll take it.
Anode used to make the solution is on it's last legs.

These are handlebar clamps from a DT250 project I haven't started... at least not in earnest yet. I bead blasted the rust off 'em about 4 mo. ago.
A couple spots reappeared, but no matter. Hit 'em with an 80G rolok sanding disk to sand the casting marks off. Then switched to a brown, then red then blue scotchbrite rolok disks. Then on to the buffer with brown rouge. If I had some white, I'd have used that too 'cause the brown left some marks... anyway, onward and upward.
Scrubbed them with dish soap, then a 5 min bath in lacquer thinner.
... then into the plating tank. Every couple minutes I stirred the solution and shook the bubbles off.
I want a nice thick plating, so I'm shooting for an hour. I'm watching closely for any signs of burning or blackening. As long as it stays bright... I'll keep going.
60 min later and here we are.
I'll take it.
Anode used to make the solution is on it's last legs.
Attachments
AndersJ
XS650 Addict
Very nice results Jim! The handlebar clamps on my XR75 were definitely on my “to plate” list. And now I know I have to!
Those are very nice results! Better than polished aluminum. 
Those are very nice results! Better than polished aluminum.
HOLY COW - Mailman says that its better than polished aluminium?
Who'd'a'thunk it?
All seriousness aside Jim - these little plating jobs are really coming out nice!
Thanks guys!Better than polished aluminum.
Yeah, the nice part is, unlike aluminum... no polishing's required. It's plated. As long as the plating holds up, it'll never oxidize like aluminum does. Shiny forever...
Few more runs of smaller stuff and before too long Jim will be scaling up his operation. Then on to headlight buckets, fork ears … fenders?
How about a nickel tank with a nice orange swoop, ala the old TT500!
How about a nickel tank with a nice orange swoop, ala the old TT500!
Nice work, Jim! "...Shiny forever..." for sure. The next best thing to polished stainless (but, who makes those?) The best of all worlds: shiny, light weight, strong, corrosion resistance. Why do you think all those old Colts were nickel plated? One hundred forty seven years later (for an 1873 Peacemaker), and they're still shiny! That bike of yours will look a million bucks!
Back in the early 70's I had a 68 Bonnie with a nickel plated frame. I'd love to do that with an XS.and before too long Jim will be scaling up his operation.

You're gonna need a bigger tank...

AndersJ
XS650 Addict
You’re a man obsessed! Excellent.
Very nice work there Jim!!
From your photo there is a lot of debris from that Anode. In that "Quite a nice little 60 -70 page help guide" they mention this. They say agitation of the solution can result in the fine debris particles settling on the horizontal surfaces of the object been plated. This leads to a rough finish. Their answer is to place the anode in a bag to retain the fines like a dust filter does with air. I do not know what material the bag is made from industrially but my guess would be to use any very fine woven synthetic cloth. With the dust retained you would be able to use more severe agitation to help knock those bubbles off.
I eagerly await that XS frame...
From your photo there is a lot of debris from that Anode. In that "Quite a nice little 60 -70 page help guide" they mention this. They say agitation of the solution can result in the fine debris particles settling on the horizontal surfaces of the object been plated. This leads to a rough finish. Their answer is to place the anode in a bag to retain the fines like a dust filter does with air. I do not know what material the bag is made from industrially but my guess would be to use any very fine woven synthetic cloth. With the dust retained you would be able to use more severe agitation to help knock those bubbles off.
I eagerly await that XS frame...
I used to do this for a living and what i see here is good backyard stuff although a little primitive compared to what i was used to though, nickel is slow any way, with regards to fines all our solutions were pumped and filtered and a lot of different chemistry to make it more efficient, as always there is more than one way to skin a cat and thinking outside of the box very often comes up trumps.
Kevski, good to see your comments regarding past experience. I realise there are substancial chemistries involved throughout the process. Unfortunately most additive components are well kept secrets. Would you happen to be aware of any simple additives/procedures for minimising bubble formation on the workpiece or to prevent bubbles sticking to the workpiece e.g. I have read that bubbles sticking is a surface energy issue, so would adding dish washing liquid release them faster?
For me the parts I would typically most want to plate are bolt and Allen key heads. Would nickel direct to steel be mechanically sound enough when tightened with a spanner or would I need to copper plate first?
Thank you for any suggests to can offer, its all good stuff to know.
Paul.
For me the parts I would typically most want to plate are bolt and Allen key heads. Would nickel direct to steel be mechanically sound enough when tightened with a spanner or would I need to copper plate first?
Thank you for any suggests to can offer, its all good stuff to know.
Paul.
As we are talking backyard stuff here and it is not mainstream chemistry it makes it difficult to recommend a cure for the problems encountered, the bubbling on surfaces is by and large hydrogen and to reduce this you need a small amount of hydrochloric acid added to the solution, without this you can finish up with hydrogen embrittlement and the whole lot of plate can just crack off the work piece, the purity of solutions is also very important, nickel plate direct to steel is the correct way to do your bolts, copper first will give you a soft sub-stratta and damage easy, and as most copper is acid based it does not take directly to steels, you would need an alkaline copper strike for this, there is not much point in recommending so called secret additives as in the UK you have to be licensed to carry or buy them which then means you have to use within regs and dispose of to a licensed waste carrier, consent to discharge rinse waters is also needed it's a minefield here, if i want shiny these days i buy new or get others to do it for me.
Thank you Kevski. This morning I checked through my junk and hope to plate a bolt or two within the week.


